Based on materials from androidcentral.com
It seems that there is nowhere to go from talking about exploding phone batteries, thanks to Samsung and their Note 7. However, this is not the first and not the only device with such a problem. Why do batteries explode at all? Let's talk in more detail …
As long as we use lithium-ion batteries, isolated cases of batteries will occur. The Note 7 isn't the first phone to have required a massive recall for battery-related reasons. For example, longtime fans of Nokia may recall something similar. But then the batteries were removable, and the Finnish company did not have to remove the models from the market. It happens, there is nothing good about it, but it happens. Let's see why.
How your phone battery works

The first thing we'll look at is how the lithium-ion battery in your device works. The essence lies in the name itself: electricity is delivered from one electrode to another through charged lithium ions.
Lithium-ion batteries store, transmit and release energy through electrochemical processes. The battery has two electrodes, an anode and a cathode. The cathode contains positively charged ions and the anode contains negatively charged ions. An electrolyte is located between the two electrodes. In a lithium battery, this is usually a natural solvent paste with a high content of metal salts (in most cases lithium) in the composition. This provides electrical conductivity. The electrolyte contains an anode and a cathode, which are separated and do not touch.
When you discharge the battery (using your phone and not charging it), the positive ions leave the anode and the negatively charged cathode attracts them. Electric current comes from the anode, travels through your device, and returns to the cathode. Yes, yes, it describes a circle, and is not 'wasted' by a charged device. When you charge your phone, the reverse process takes place: ions move from the cathode to the anode through the electrolyte.
When these ions come into contact with charged atoms on an electrode, a redox electrochemical reaction occurs, which releases charged electrons that pass through the battery contacts connected to the electrodes. Due to this, the lithium ions in the electrolyte continue to charge until there are not enough of them to hold a sufficiently strong positive charge to move through the electrolyte, at which point the battery stops charging.
Lithium is the lightest metal, number three on the periodic table. It also easily reacts, which makes it very easy to cause this reaction. Thus, it is almost ideal metal for use in a portable reusable battery. It is lightweight, easy to recharge and can hold a charge for a long time.
What Causes Batteries to Explode?

First, let's define what in this case is understood as an explosion. The electrolyte paste inside a lithium-ion battery is highly volatile. It can easily react with other metals and has a very low melting point (180 degrees Celsius). Inside the sealed battery case, pressure can build up to the point of breaking the integrity of the case, and then quickly break out. The pressure expels very hot electrolyte, which could cause a fire. Some lithium batteries have an air vent so they will not collapse from pressure. When the battery case collapses and the superheated liquid containing molten metals is forced out under pressure, it causes an explosion.
There are two ways to make a lithium battery explode – overheating and physical damage. Let's consider both of these methods.
Overheating and overcharging
This is the most common cause of battery problems. During the charging process, something is disturbed and the incoming voltage continues to support the chemical reaction. One section of the battery becomes too hot, and as charging continues, it cannot cool down, and what is called thermal runaway occurs.
In this case, the hot section begins to generate its own heat, which overheats the electrolyte in the neighborhood, and this, in turn, overheats other parts of the battery. The heat is spread by the electrolyte and forms vapor, raising the pressure until the battery case cracks, venting the accumulated pressure and hot, sticky and highly flammable liquid on contact with air.
When this happens, it can cause physical damage to anything close to it – boards and glass or plastic. These materials can also ignite when heated, which in turn ignites the leaked electrolyte, making it something like napalm that sticks and burns through the surface or burns itself.
The thermal runaway process can occur very quickly, and the situation from the discharge of the norm can go to catastrophic inside the battery even before the heat is transferred from the apparatus to your hand. Fortunately, the hundreds of millions of lithium batteries produced annually have an extremely low (statistically almost insignificant) failure rate due to thermal runaway, due in part to safety measures (such as non-flammable additives in electrolyte and coatings).
When your phone gets too hot and says it can't charge or run at full power, it needs cooling to prevent thermal runaway. Listen to the small pop-up message and let the device cool down.
Mechanical damage
Lithium batteries are specially designed to be lightweight, high performance and easy to charge. This means that the outer shell and the separation separating the electrodes are very thin and light. Most of the weight comes from components that are directly involved in charging the phone.
Since the baffles and battery casing are thin, they can easily be torn or punctured. If the battery is damaged so that the electrodes can touch, a short circuit may occur. An instantaneous electrical discharge is explosive and can heat up the electrolyte and create pressure that will explode through any damage to the battery case. Hot, flammable, contact with a spark occurs – here's a recipe for disaster.
The thin body, oddly enough it sounds, is also a precautionary measure. The thinner the metal, the easier it is to damage it, due to this, such a large pressure does not form, a kind of ventilation hole is obtained. It is bad when a hot flammable liquid bursts out, but even worse when pressure builds up inside a thicker case, until it finally destroys it.
Other metals in contact with the electrolyte paste can also cause a spark that will explode. You can search YouTube for videos yourself in which very smart comrades pierce phone batteries to explode. The reaction to contact with foreign metal will be a short circuit, but on a smaller scale.
What about the Note 7?
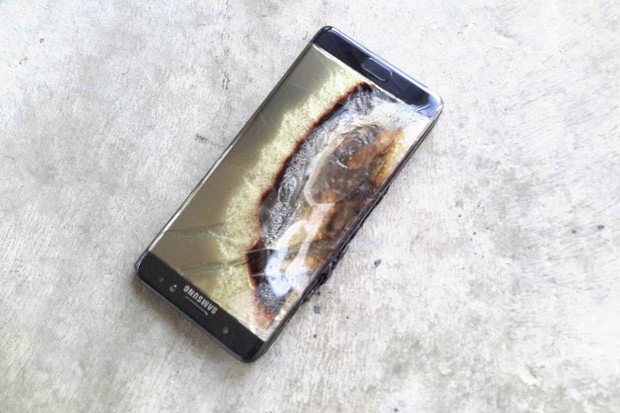
Only Samsung knows for certain why the batteries in the Galaxy Note 7 explode. The company sent out a short message in its UK division that doesn't make much sense. The phrase 'the anode-to-cathode came into contact' sounds like it is describing a short circuit, but it doesn't mean anything like that. But being a couch expert who has never seen an exploding or already exploded Galaxy Note 7 with his own eyes, we can say that the most likely situation is the anode-to-cathode closure.
Since people have reported explosions of Note 7 batteries not being charged, and a significant portion of the phones we see in the photo did not burn, it can be assumed that thermal runaway is not to blame, despite the fact that Health Canada accurately states that a single case in Canada came from overheating.
Thermal runaway is not as 'instantaneous' as a short circuit, and it takes longer for a heated electrolyte to burst out of the battery with a flame than it does to explode out of its housing. Will burn more than a part of the phone, as well as other objects in the immediate vicinity. Also, a thermal explosion will cause the battery to swell before the integrity of its flexible body is compromised, and without further space to expand, the swollen battery will break the cover of the phone itself. Videos on YouTube demonstrate how this happens, here is a good old example of someone breaking precautions to make it happen. Someone who was probably told the phone would swell before exploding.
It can hardly be assumed that any foreign bodies or particles that have got into production come into contact with the electrolyte. This is a popular hypothesis, but if a batch of devices (we don't know what size the batch) contains small particles in the electrolyte, we will see many more Galaxy Note 7 exploding.
It seems more likely that there is a short circuit inside the battery or in the charging circuit, coupled with an electrolyte that has non-flammable additives. A rapid explosion that releases a small amount of pressure and liquid at the same time can exhaust itself if nothing is in contact with the apparatus. If there is contact with something combustible, for example, with the same jeep seat, a fire can occur.
Of course, there can be a lot of factors that we are not aware of. The manufacturing process of lithium-ion batteries for phones is highly safe. But a lot can go wrong.
Samsung's preliminary report to Korean regulators cited a manufacturing error that caused the circuit boards inside the battery to contact each other, causing “excessive heat.” The report said a more thorough analysis was needed to figure out the exact cause.
The preliminary findings point to a manufacturing error that puts pressure on the circuit boards in the battery cells. This in turn forces the positive and negative poles to come into contact, causing an excessive temperature rise. However, Samsung is focusing on the need for a more thorough analysis of the situation to determine the 'exact cause' of battery damage.
What do we know about the Galaxy Note 7 and its battery

We have no idea what's going on with the Galaxy Note 7, nor does anyone outside of Samsung. But we can make an educated guess based on what little knowledge we have. The Galaxy Note 7 has been recognized as a problem device by Samsung, has been phased out and we must stop using it and return it. Actually, in order to get rid of a potential source of danger, we do not need to know exactly why the devices explode. What to do is up to you, as well as how to treat the situation as a whole. We just tried to lift the veil over the technical side of the issue.
