Based on materials from androidcentral.com

Each of us noticed how big the difference is between how a brand new smartphone holds a charge and how a device that has lived a year or more. And if your device has been serving you for a long time, it may not live on a single charge and a full day. Why it happens?
Battery: how does it work?
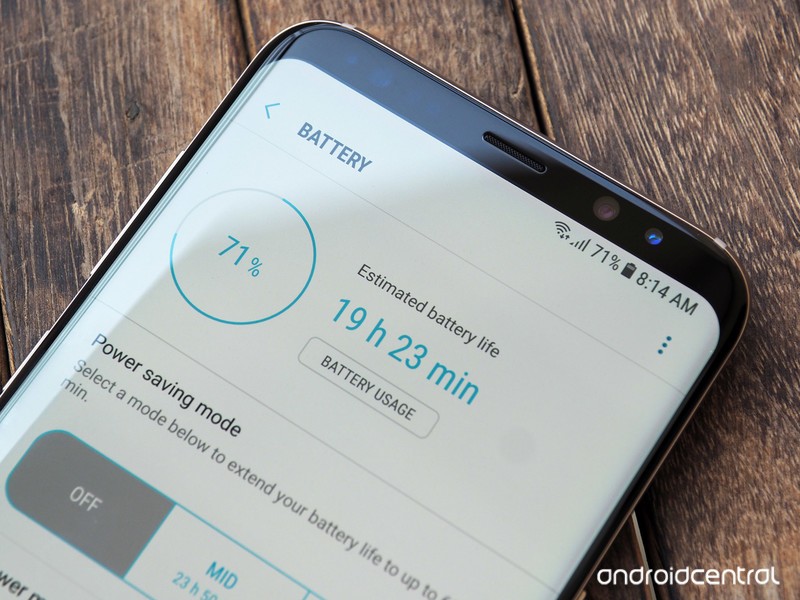
There is no magic here, only electricity. In order to understand why now you need to charge your smartphone more often, you first need to understand in the most general terms how the battery works in general.
Electricity, like any energy, is not something that you can just take and create. What we call 'producing electricity' is just the transfer of one type of energy to another. A battery uses a chemical reaction to create an electrical charge that can be measured over time. Various materials can be used to create this charge, and they will give different results. Our smartphones use lithium-based batteries because they produce enough energy at an affordable cost.
Inside the smartphone's battery, you will find three components that are important for our conversation. This is a negative electrode (called an anode and is usually made of graphite), a positive electrode (called a cathode and is made of an alloy of lithium and other metals), and an electrolyte solution. The reaction that occurs with the participation of these three components is simple in its essence and allows you to use them for the accumulation of energy. When you charge the electrodes (while using the charger), the lithium ions are positively charged and are attracted to the negatively charged electrode. When you take your smartphone out of charge, these lithium ions begin to lose their positive charge and stop being attracted to the negatively charged electrode. The longer you drain the energy of a charged battery, the more the amount of lithium ions that are devoid of charge increases. This happens until the moment when there are no charged ions left in an amount sufficient for the battery to produce at least some energy, and then it turns out to be discharged. Then we put the smartphone on charge again and the cycle repeats.
The 'cycle' is a very important concept here. Batteries are designed to store energy and it is very difficult to measure their life in units of time. For example, your battery will last two years, while someone will only have six months, because it will be used in different ways. So we can only assume how long the battery will live, and this period will be measured in charge cycles. A typical smartphone battery is designed to withstand about 500 to 600 cycles (a cycle is defined as charging a fully discharged battery to 100% and then discharging it to zero). Charging a half-discharged battery, and then discharging it to 50% is an incomplete cycle, which is why you are advised to charge your smartphone before it is discharged to zero, and you are also advised not to try to fool the system in this way and delay the onset of the 500th cycle. Of course, this does not work that way, since your battery does not count charge cycles. Five hundred is a rough estimate.
However, lifespan can be measured in cycles, taking into account what happens when you charge the battery and how this affects subsequent charge cycles, the amount of energy the battery can store, and potential voltage.
Sworn enemies – oxidation and energy efficiency
Electric cars are already a reality, and the batteries they use are insanely expensive. Therefore, there has been a lot of research on why lithium-ion batteries degrade over time. The results apply to the less expensive (but still not cheap!) Batteries in our smartphones. And the culprit is the chemical changes that occur during charging.

So, we know that when the battery is charged, lithium ions get a positive charge and are attracted to the negative electrode. As more and more charged ions are attracted, the potential difference between the positive and negative electrodes, known as voltage, increases. When it reaches a certain value, the battery is considered fully charged. When the battery is discharged, the opposite process occurs: the potential difference decreases until it reaches zero, since there are no more positively charged ions left on the negative electrode. But this does not mean that the negative electrode remains the same as it was before the start of the process.
The electrodes are oxidized. Likewise, water and air make iron rust. Lithium, graphite and electrolyte salts cause the electrode to oxidize. When each positively charged ion is separated from the anode, it leaves behind a microscopic layer of particles that is chemically bonded to the graphite anode. These are particles composed of atoms of lithium oxide and lithium carbonate, none of these substances have the same chemical or electrical properties as graphite. This layer interferes during the charge / discharge cycle by varying the potential difference (voltage) and the amount of charged ions that the electrode can attract. Obviously, the changes will be noticeable. If you keep using the battery and charging it as you normally would, there will come a point where it cannot store enough power to turn on your smartphone.
Different types of lithium alloys, like the different salts that are used in the electrolyte solution, affect how many of these particles remain on the electrode, but materials that provide a cleaner cycle are not necessarily the best because they cannot provide sufficient accumulation. energy. In smartphones, we need batteries with high capacity and low power consumption, because they are safer and less expensive than more powerful batteries, and we need them to power our devices for as long as possible. In electric vehicles, you can use very capacious and very powerful batteries, since they are protected by a durable case, they are less likely to be damaged, and the car will need to travel a long distance between charges. However, the cost of replacing the battery for the Tesla Model S is $ 12,000. Part of the reason for this price lies in the cost of the materials required to make a lithium-nickel-cobalt-aluminum-oxide battery, as opposed to a conventional lithium-cobalt battery in a smartphone, which is not designed for the same number of cycles.
Voltage matters
One of the most important factors affecting the number of cycles a lithium-ion battery can operate is its voltage. Phones and cars are not the only area of application for lithium batteries, in the US in 2015 the Department of Energy spent a lot of time and money to understand how the problems are connected and how to solve them – after all, satellites use lithium-based batteries and solar cells. And in addition to the design of the battery, the nominal charge voltage and the nominal voltage of the battery itself turned out to be a negative factor affecting the service life.
The chemical process by which the lithium battery works leads to degradation of the anode, as mentioned above. But if you charge a battery with a voltage greater than 3.9 V, a similar degradation occurs with the cathode, which will halve the battery life. Charge voltage and battery voltage are essentially the same because all components of the battery are energized, and in addition, charging causes heat, and the higher the charge voltage, the more heat will be. The heating that occurs when the battery driving voltage exceeds 3.9 V increases the cathode degradation.
In other words, the voltage it takes for a modern phone to quickly charge its battery makes the problem insoluble. Anyone who uses a battery powered drill can see this. The 12 or 14 volt batteries in the instrument don't even come close to living the same as those used in our phones. They have higher voltage, charge at higher voltage, they are much hotter and deteriorate noticeably after just a few cycles. These are the same simple lithium-based batteries found in the phone, because using materials like the Tesla S batteries would be too expensive and would not last long. Fortunately, most of the materials are recycled and we don't drown in power tool batteries, where lithium is more expensive than gold.
The good news is that lithium battery companies are trying to improve the situation. Anyone who manages to create a battery that can last significantly longer will make a lot of money. And we just have to charge our phones when they need it, and understand that there is no conspiracy of battery manufacturers to force us to buy new devices more often.
